On February 17th (EST), 2022 American Society of Clinical Oncology Symposium on genitourinary urogenital neoplasms(ASCO-GU)was held, RemeGen(荣昌生物-B:09995.HK) announced its latest progress of clinical research on the treatment of locally advanced or metastatic urothelial carcinoma with Disitamab Vedotin combined with toripalimab, the research data showed good efficacy and controllable safety.
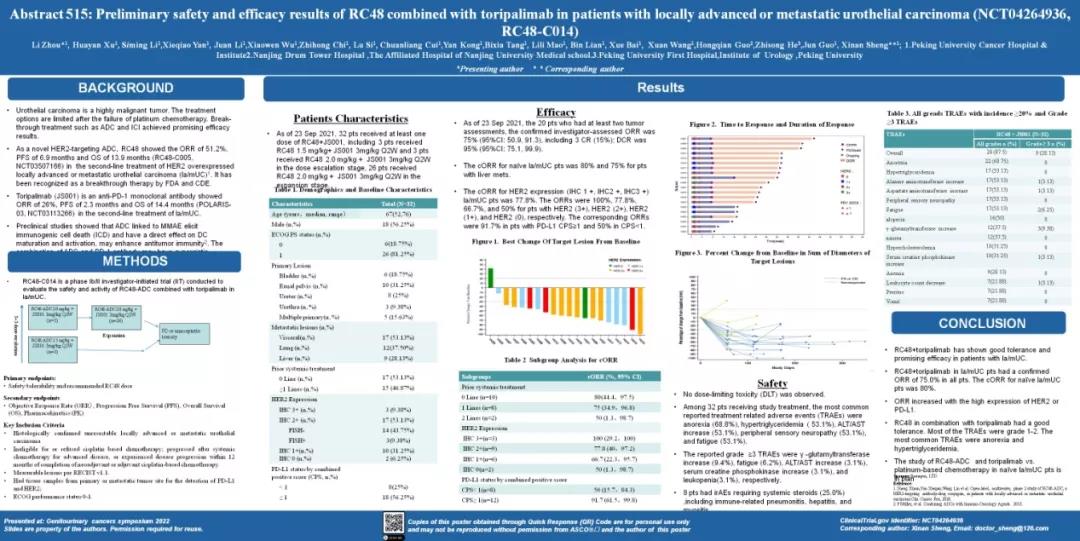
The poster showed preliminary safety and efficacy results of RC48 combined with toripalimab in patients with locally advanced or metastatic urothelial carcinoma (RC48-C014), led by Prof. Jun Guo of Beijing Cancer Hospital, Prof. Xinan Sheng and Prof. Li Zhou were the principal investigators. According to the latest data, the confirmed investigator-assessed ORR was 75%, the cORR for HER2 expression (IHC 1+, IHC 2+, IHC 3+) la/mUC pts was 80%.
These researches showed that patients benefited from the combination of Disitamab Vedotin and toripalimab regardless of treatment line number and HER2 and PD-L1 expression status, and that ORR increased with high HER2 or PD-L1 expression. What was particularly surprising was that the objective response rate (ORR) in HER2(3+) patients is as high as 100%. This outstanding efficacy demonstrates the success of the combination therapy concept of ADC+ PD-1 mab, but also is expected to be a major breakthrough in the treatment of urothelial carcinoma. The long-term survival benefits of PFS and OS from this research deserve continued attention. Currently, researches on Disitamab Vedotin combined with toripalimab versus platinum-based chemotherapy are being actively prepared.
