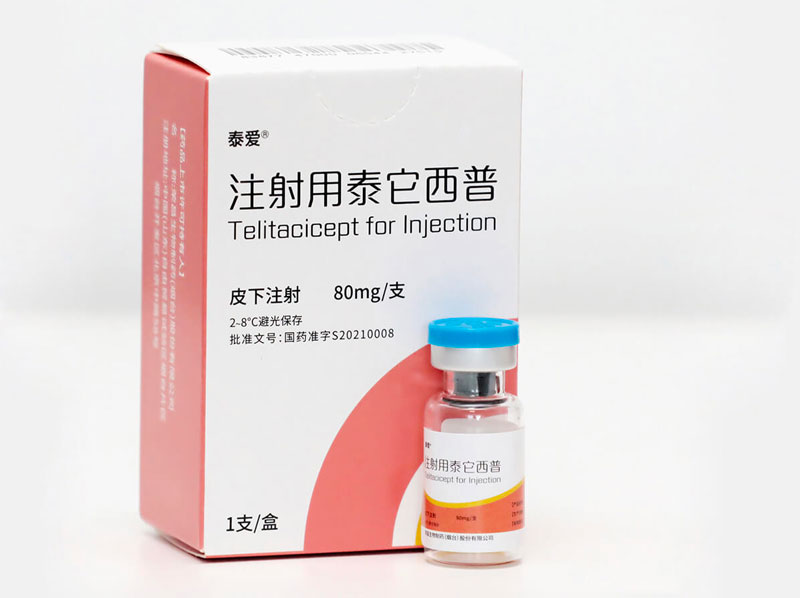
Telitacicept
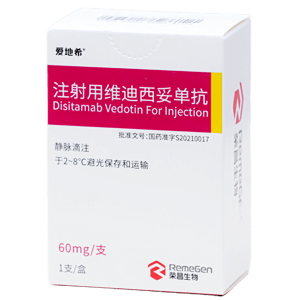
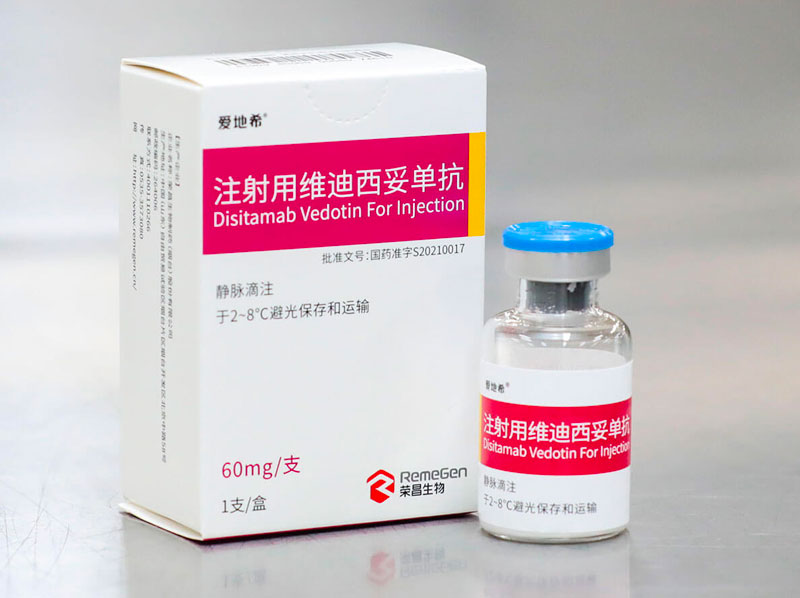
Disitamab Vedotin
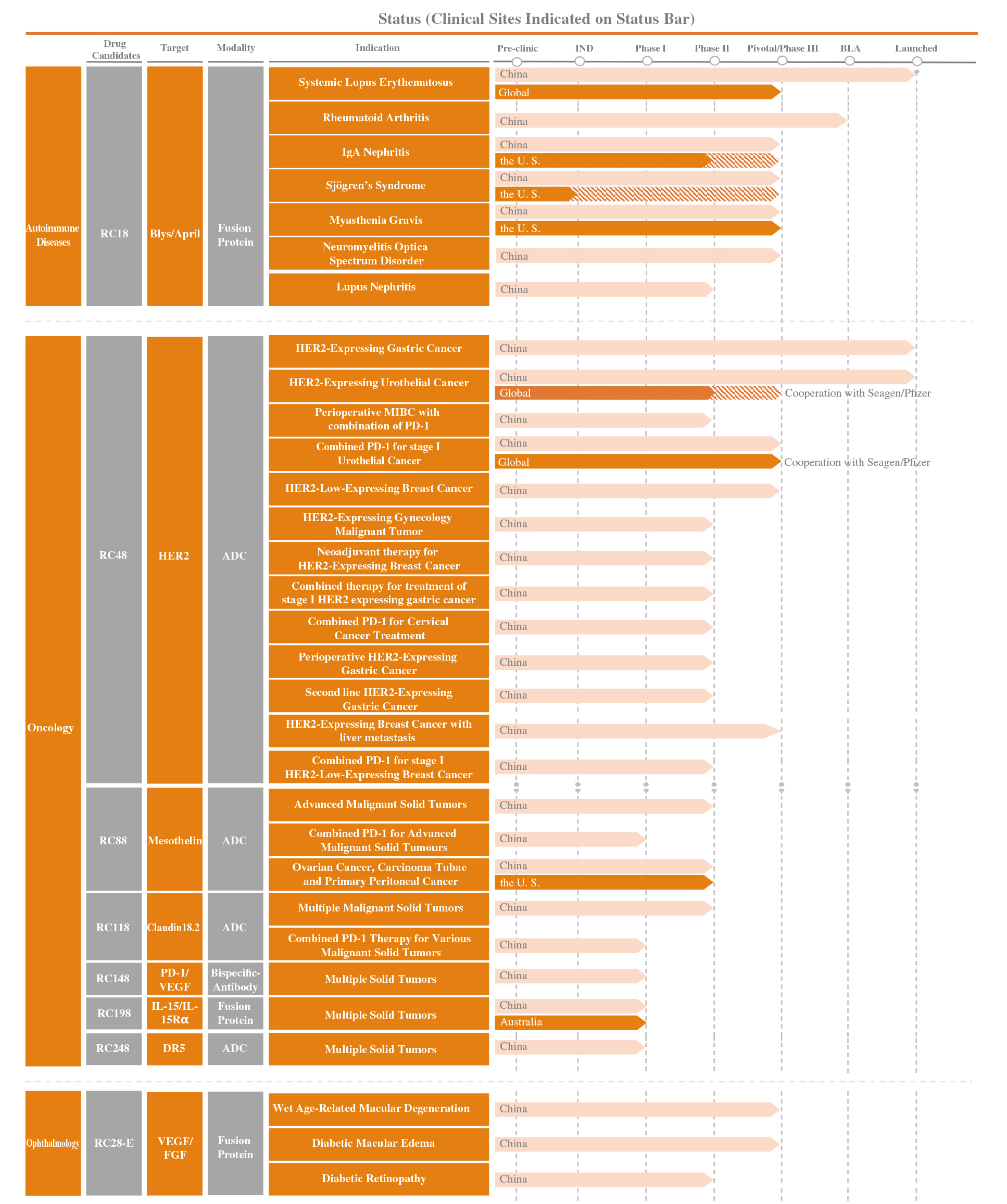
Product Pipeline
- Oncology
- Autoimmune Diseases
- Ophthalmology
Production and Manufacturing
We have state-of-the-art production facilities that comply with global GMP standards. They meet the production needs of the company’s product pipelines in the clinical and commercial stages. There are also advanced disposable bag bioreactors. We already have the competitive strength to produce a variety of innovative biopharmaceutical products on a large scale, and at the same time, we are building new production facilities.
Quality Assurance
RemeGen has integrated production capacities to produce monoclonal antibodies, fusion proteins, antibody-drug conjugates, bifunctional antibodies and other complex molecules. Its factory buildings and the ancillary facilities have reached world leading. We have built a professional quality assurance team led by medical professionals with decades of biopharmaceutical development experience in Europe or the United States. We have been focused on building a quality management system that meets the international standards to expand our global outreach. RemeGen have established the quality policy of Making Medicines With Integrity, Scientific Management,Continuous Improvement and Pursuit of Excellence. We shall build a quality management system that meets the GMP requirements of FDA, EMA and NMPA.