News
-
17 2025.06
RemeGen's Telitacicept (RC18) Received Orphan Drug Designation from EMA for Myasthenia Gravis
-
27 2025.05
RemeGen’s Telitacicept Approved for Treatment of Myasthenia Gravis in China
-
09 2025.04
Remegen Announces Exciting Results of Telitacicept Phase 3 Clinical Trial for Patients with Generalized Myasthenia Gravis
-
27 2024.10
Priority Review Granted to BLA of RemeGen’s Telitacicept for Its Third Indication of Myasthenia Gravis
-
13 2024.08
BLA on Agenda: A Phase III Trial on Telitacicept for Myasthenia Gravis Achieves Primary Endpoint in China
Mechanism of Action
Telitacicept is a proprietary novel fusion protein of us to treat autoimmune diseases. It is constructed with the extracellular domain of the human transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptor and the fragment crystallizable (Fc) domain of human immunoglobulin G (IgG). Telitacicept targets two cell-signaling molecules critical for B-lymphocyte development: B-cell lymphocyte stimulator (BLyS) and a proliferation inducing ligand (APRIL), which allows it to effectively reduce B-cell mediated autoimmune responses that are implicated in several autoimmune diseases.
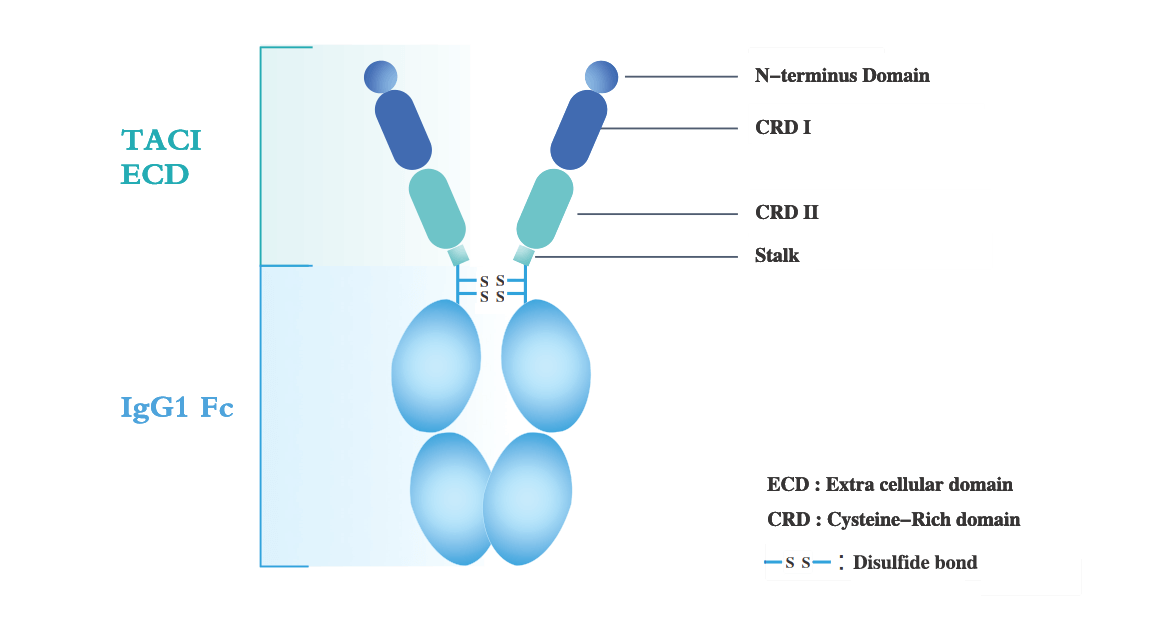
BLyS (also known as B-cell activating factor, or BAFF) and APRIL are both involved in the development of B cells from pre-B lymphocytes to mature B cells, and ultimately to plasma cells, the professional cells producing antibodies, as well as in the co-stimulation of T-cell proliferation under certain conditions. Aberrant B cell activities and antibody production are known to be implicated in a number of autoimmune diseases. BLyS and APRIL function through the following mechanisms:
• BLyS binds to three types of membrane receptors expressed on B-cells, i.e., TACI, B-cell maturation antigen (BCMA) and B-cell activating factor receptor (BAFF-R), to inhibit cell death and stimulate differentiation of B cells into antibody-producing plasma cells. The interaction between BLyS and TACI induces a T-cell independent B-cell activation, immunoglobulin class-switching and B-cell homeostasis, while BLyS’ interaction with BCMA is important for the differentiation and survival of plasma cells.
• Unlike BLyS, APRIL only binds to TACI and BCMA (but not BAFF-R) to modulate the function and survival of B cells and promotes their differentiation into plasma cells.
• In sum, whereas BCMA binds to BLyS weakly and BAFF-R does not bind to APRIL, TACI binds to BLyS and APRIL with equal affinity and can also bind to heteromeric forms of BLyS and APRIL.
• BLyS and APRIL also play a role in the co-stimulation of T cells as B cells and T cells cross-talk. For instance, since BAFF-R is a potent T cell co-stimulator, the signalling of BLyS to BAFF-R could promote aberrant T cell maturation, which is known to be implicated in certain autoimmune diseases.
Consistent with their known functionalities, increased BLyS and APRIL expression has been observed in various B cell-mediated autoimmune diseases, such as SLE, NMOSD and RA. Studies have shown that direct inhibition of BLyS and APRIL has the potential to prevent the engagement of their receptors, BAFF-R, TACI and BCMA, and thus to prevent the subsequent activation of B cell-driven mechanisms, such as autoantibody production that contributes to the pathology of autoimmune diseases. BLyS and APRIL have therefore emerged as important targets for autoimmune therapeutics, although most of the clinical-stage drug candidates targeting this signaling pathway have been designed to neutralize either BLyS or APRIL, but not both.
As illustrated in the diagram below, Telitacicept blocks BLyS and APRIL from binding to BAFF-R, BCMA and TACI receptors expressed on B-cell surface, suppressing the BLyS and APRIL signaling, and inhibiting the development and survival of mature B cells and plasma cells.
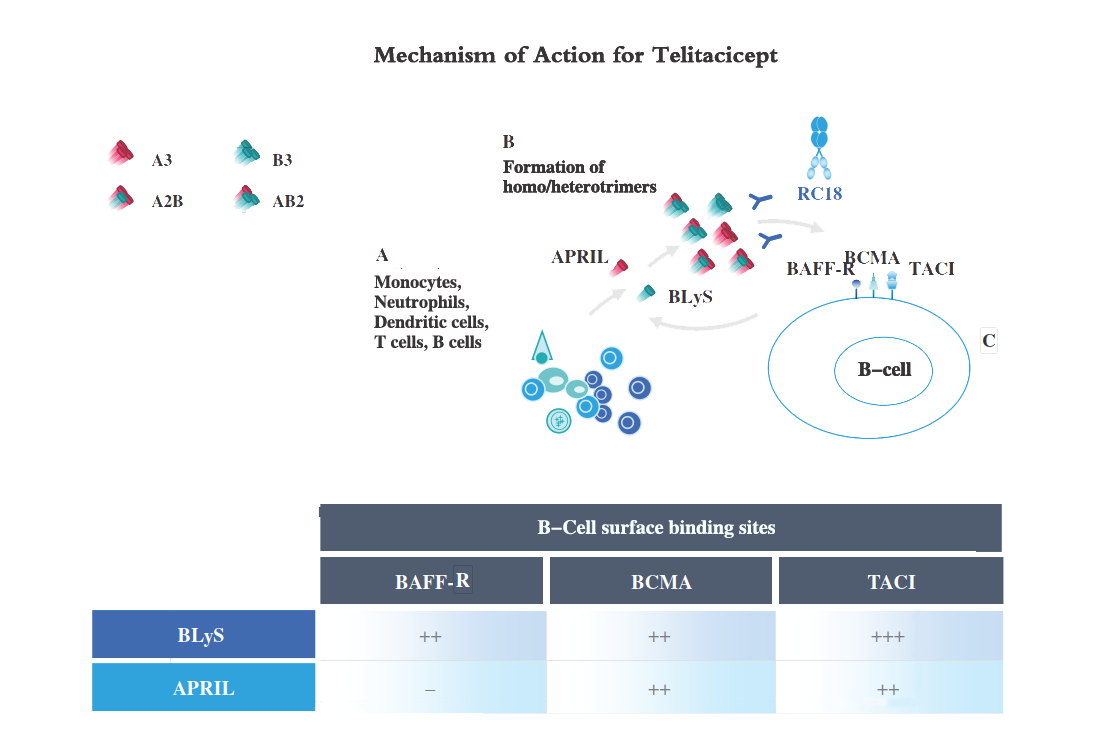
Abbreviation: A3 = APRIL homotrimers; B3 = BLyS homotrimers; A2B = heterotrimers of two APRIL and one BLyS molecules; AB2 = heterotrimers of one APRIL and two BLyS molecules.
Indication
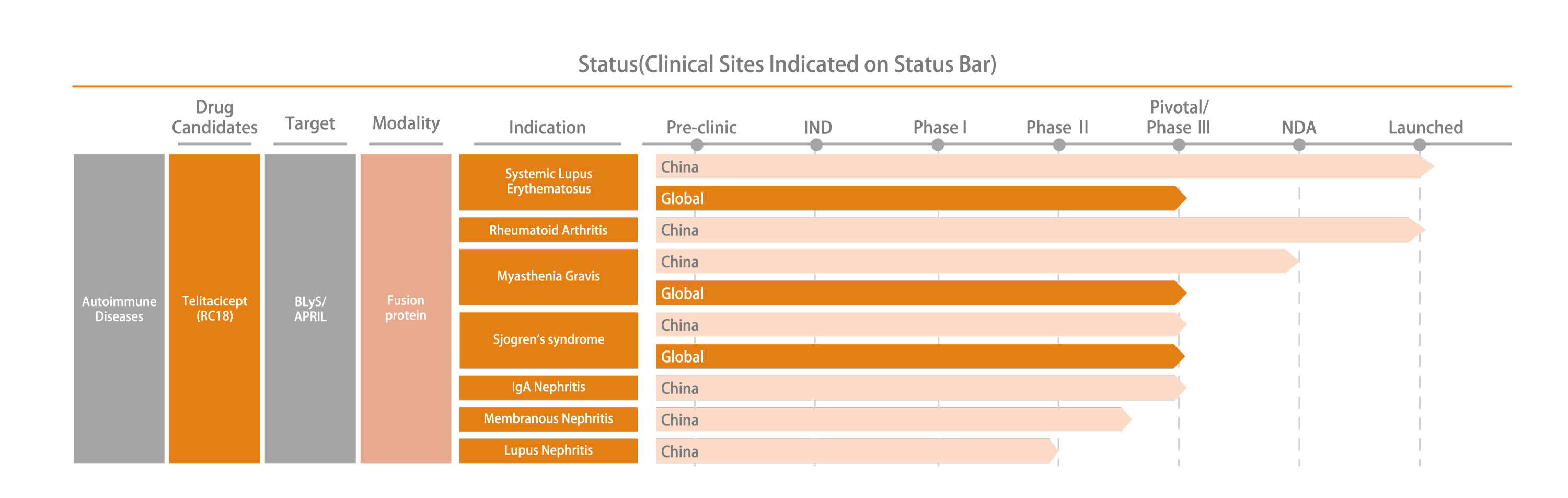
Telitacicept is the first biological drug for dual-target treatment of systemic lupus erythematosus in the world. In March 2021, the new drug marketing application of Telitacicept for the treatment of SLE passed the priority review and approval process. As an urgently needed clinical drug with outstanding clinical value, it was conditionally approved for marketing in China and entered the National Reimbursement Drug List. In November 2023, Telitacicept was formally granted full approval from conditional approval by China's National Medical Products Administration (NMPA).
In addition to SLE, we are actively developing Telitacicept for the treatment of neuromyelitis optica spectrum disorders, rheumatoid arthritis, IgA nephropathy, Primary Sjogren’s syndrome, relapsing-remitting multiple sclerosis and systemic myasthenia gravis and other B cell-mediated refractory autoimmune diseases with unmet clinical needs.
Competitive Advantages of Telitacicept
• Structural design Advantages
- Unique two-target mechanism improves blocking effect
- Good biological activity
- Better stability and extended half-life
- Optimized immune tolerance
- Low immunogenicity
• Production advantages
Humanization and molecular structure design optimized by molecular informatics effectively improve the molecular stability of Telitacicept, prolong the half-life, and making it more suitable for large-scale industrial production. We have built a fusion protein production facility in compliance with GMP standards, realized the stable and efficient production of fusion protein products, which can meet the clinical and commercial production requirements.
• Preclinical and clinical advantages
Based on its unique two-target design and bioinformatics optimized protein structure, Telitacicept has demonstrated excellent efficacy and safety in preclinical and clinical trials, with no drug-related immunogenicity observed in clinical trials.